It is from water can be obtained hydrogen fuel, in principle, not giving the oxidation of harmful products. Reacting with oxygen, the hydrogen is again converted into water.
Thus, this chemical element is, apparently, the only fuel that is converted during combustion into a product from which it had once received.
The use of hydrogen as a motor fuel or as additives to hydrocarbon fuels is not new. So, during the great Patriotic war, the siege of Leningrad and in Moscow there was a car working on hydrogen, used at that time for barrage balloons. An in-depth study of the working process of piston engines on hydrogen-air mixtures has received in our country, the development in the postwar years. The experiments conducted on a spark ignition engine showed that the flame when burning hydrogen is distributed in the combustion chamber of the engine six to ten times faster than the feed of the hydrocarbon fuel. The engine can operate stably in the hydrogen fuel even with a significant lean condition (sa = 2-3). This lean mixture allows you to skip to the good regulation of the engine, i.e. at a constant amount supplied to the engine air change only the content of hydrogen.
For ignition of hydrogen mixtures requires a small, 12 times less than for gasoline, ignition energy. Due to this, the requirements for ignition systems can be significantly reduced.
If before the working process research of hydrogen internal combustion engines (VDS) was mainly of a theoretical nature, but now these works are going into practical technology. The scope of searches involving the use of hydrogen is the fact that in recent years, the United States experimental study WDS conducted at fifteen academic centers. Tested 42 of the engine, including in the testing conditions three rotary piston. Built 15 vehicles with engines on hydrogen. In our country creation of cars running on hydrogen fuel are engaged in the Institute for mechanical engineering problems, Academy of Sciences of the USSR, similar studies are carried out in Leningrad.
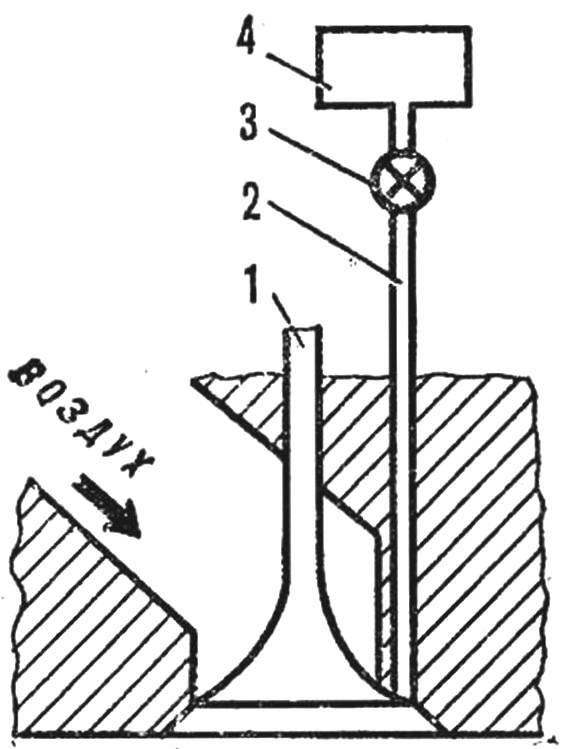
As it turned out, for VDVS most preferred is the internal mixture formation. Hydrogen is supplied to the intake valve seat (see picture) and is mixed with air when it is opened. Here is regulated only by the supply of hydrogen. When using such a mixture the engine runs without detonation at compression ratios up to 19.
The contents in the exhaust gas hydrocarbons and carbon monoxide is negligible. Thanks to the use of lean mixtures, the engine efficiency can be increased by about 25%. In what form can be stored and transported hydrogen car? The most simple and reliable compressed gas cylinders, checked during the operation on natural compressed gas. However, the existing cylinders because its gravity is not suitable for transporting hydrogen, which have low density.
Fig. 1. Diagram of air intake and hydrogen in the engine:
1 — intake valve, 2 — channel for supplying hydrogen, 3— throttle, 4 — tank with hydrogen.
The use of liquefied hydrogen is much more complicated and more expensive. However, the use of tanks with liquid hydrogen brings the following system by the specific gravity to the existing power units of vehicles.
Of practical interest can be hydrides of some metals. They play the role of a hydrogen storage. When thermal decomposition of the hydride produces hydrogen. Used metal can be re-saturated by hydrogen.
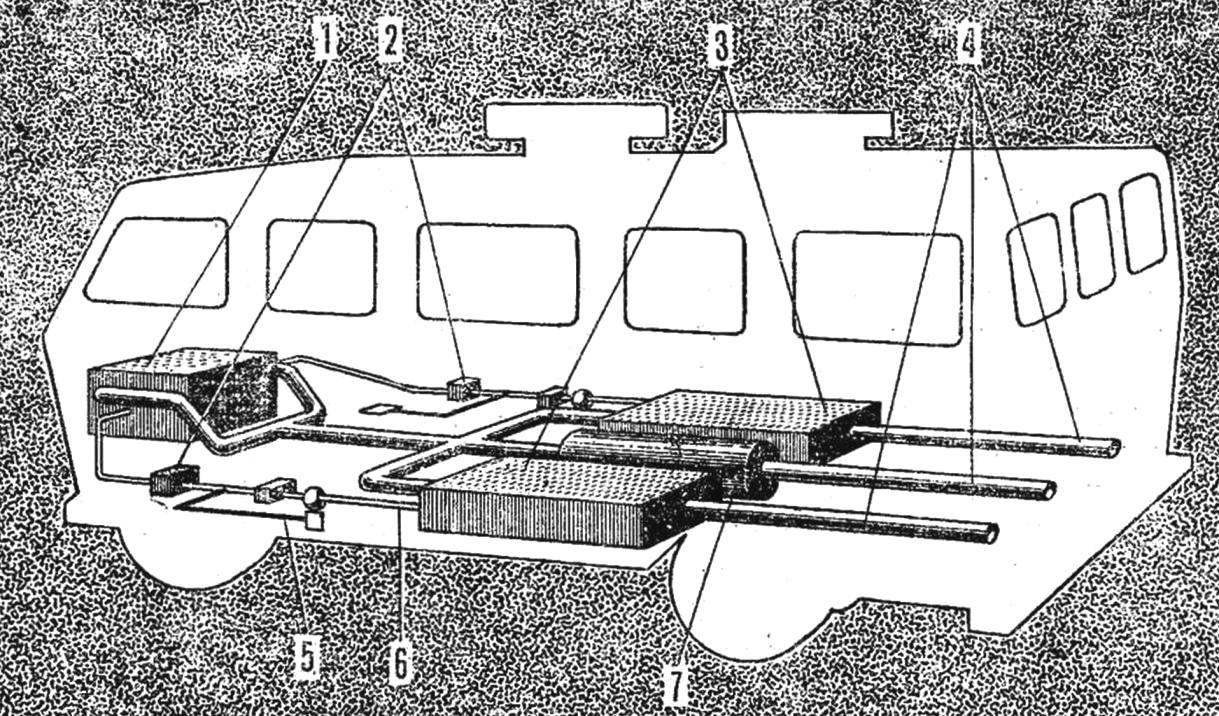
One such hydride is titanium hydride geletey (ТiFeН2). The figure shows the layout of the engine and hydride tanks on the bus one of the foreign firms. The range of this bus on one tank filling is about 180 km. Used hydride easy it is to refuel with hydrogen in only 5-10 min depending on the pressure of supplied hydrogen. The amount of hydride in the same tank is around 50 litres but it holds 50 thousand litres of hydrogen gas, the pressure in the tank is not more than 2 ATM.
To obtain the gaseous hydrogen from the hydride needs to be heated. Heat can be derived from both engine cooling system and through air-conditioning in the car. Even when the engine is ka idle energy consumption of the hydride tion of the tank is about 4 thousand calories per hour. In other words, this amount of heat must spend for the isolation of the hydride hydrogen to satisfy the demand of the engine at idle. At full load the energy consumption of the hydride reservoir increases by 10 times. Starting work is accomplished without external heating of the hydride tanks, as even at -20° C generated a sufficient amount of hydrogen.
However, the hydride system by weight are on the level of modern compressed gas cylinders. So, every tank together with the metal hydride weighs 680 kg, and two — 1380 kg, and the total weight of the bus 7257 kg.
Upon receipt of the hydrogen required for saturation of these pools, and low-grade coals the cost is only slightly higher than that of high-octane gasoline. It should be noted that in connection with the depletion of natural reserves of liquid hydrocarbons, gasoline prices will increase. At the same time as improving the technology of producing hydrogen, its cost should fall. Much greater difficulties are associated with the creation of a widespread network of delivery and distribution of hydrogen. In addition, although the price per unit of weight of the hydride and low, but it will need a huge amount in mass transit vehicles on the hydride system.
Fig. 2. Diagram of a car with a hydrogen engine:
1 — engine, 2 — pressure regulator; 3 — tanks with hydride, 4 — exhaust pipe 5 — gas line, 6 — submission of hydrogen in the engine, 7 — damper.
Known systems of some foreign companies, where the hydrogen is obtained directly on the vehicle as a result of chemical reactions.
One of them involves submitting to one half consumed by the gasoline engine in a special reactor where the produced gas of the following volumetric composition: of 0.26 — H2; of 0.236—; 0,011 — CH4; the remainder consists of inert gases. For the conversion of the gasoline into gas in the reactor takes the heat component 20% relative to the heat contained in the flowing through the reactor gas. Despite this, the overall efficiency of the engine running on a mixture of gasoline and gases from the reactor, 20% higher than when operating on gasoline. The exhaust emissions of the engine with the reactor and several times less than the petrol variant.
Another such system is based on the following reaction: in a reactor containing water and methanol in a molar ratio of 1:1, in the presence of catalyst and heated to 100-250° C the reaction СН30Н+H20 → 3Н2+CO2. The hydrogen produced will lay down additive to gasoline.
Interesting way of producing hydrogen directly into the combustion chamber of the engine proposed by two British inventors. The engine is equipped with reservoir of molten alkali metal (Na, Li, etc.). This metal is pumped to the nozzle installed in the cylinder head of the engine. The engine is also equipped with a water tank. Water enters the float chamber of the carburetor of the elementary and through the spray hits the diffuser, where it is mixed with air. Air-water mixture enters the engine cylinder at the intake passage provided with a valve.
In the combustion chamber reaction occurs between the molten alkaline metal and water. The resulting hydrogen ignites from compression. Water vapor is discharged into the atmosphere through the exhaust valve and muffler.
Formed in the reaction process, the hydroxide of the metal is drained from the cylinder tube, the inlet of which is located in the cylinder wall at a distance of about 1/3 of the piston stroke from TDC. Hydroxide in the atmosphere reacts with carbon monoxide, forming harmless carbonate.
Reaction of alkali metals can be performed in a separate enclosed tank with a supply of water. In the presence of a sealed reservoir formed in the reaction of the hydrogen is fed under pressure to the engine.
Thus, currently being developed by a large number of systems (including “exotic”) power a hydrogen internal combustion engines. Apparently, in the next 10-15 years there will be production models of vehicles or pure hydrogen, or using it as an additive. But the new gives rise to new difficulties. We can assume that the question arises, where to put the water produced as a product of combustion of hydrogen. If in summer, the water will go into atmosphere, then imagine what will become of the street on a cold winter day? That is, after solving the basic problems arise not less difficult task of disposing of the water.
I. ZINOVIEV, engineer
 OR AGAIN ABOUT THE ENGINE RUNNING ON THE WATER. Dmitri Ivanovich Mendeleev at the time, said to burn the oil all the same what to heat the furnace Bank notes. Since then has greatly increased the number of “ovens” which continues to burn the “black gold”. Modern kilns are, in particular, internal combustion engines, using a great number of products of oil refining; gasoline, kerosene, diesel fuel. But nowadays, with the development of synthetic materials are a large consumer of petroleum becomes chemical industry. In addition, when burning oil, the atmosphere is polluted with harmful substances. Scientists around the world are forced engine builders to develop a system of neutralization of harmful components of exhaust gases, i.e. to be like the hero of the novel, Shelley’s “Frankenstein”. As you may recall, he created with his own hands a monster, until the end of his days he was forced to fight him. Meanwhile, our planet is the substance (and its reserves are truly inexhaustible!), from where you can get fuel for all energy and transport facilities present and future. This substance— we all know the water.
OR AGAIN ABOUT THE ENGINE RUNNING ON THE WATER. Dmitri Ivanovich Mendeleev at the time, said to burn the oil all the same what to heat the furnace Bank notes. Since then has greatly increased the number of “ovens” which continues to burn the “black gold”. Modern kilns are, in particular, internal combustion engines, using a great number of products of oil refining; gasoline, kerosene, diesel fuel. But nowadays, with the development of synthetic materials are a large consumer of petroleum becomes chemical industry. In addition, when burning oil, the atmosphere is polluted with harmful substances. Scientists around the world are forced engine builders to develop a system of neutralization of harmful components of exhaust gases, i.e. to be like the hero of the novel, Shelley’s “Frankenstein”. As you may recall, he created with his own hands a monster, until the end of his days he was forced to fight him. Meanwhile, our planet is the substance (and its reserves are truly inexhaustible!), from where you can get fuel for all energy and transport facilities present and future. This substance— we all know the water.