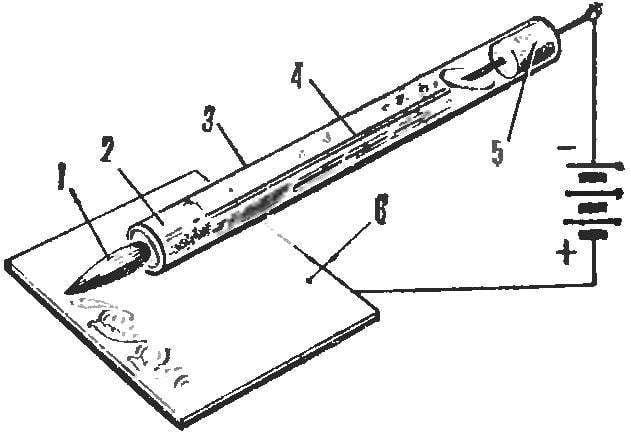
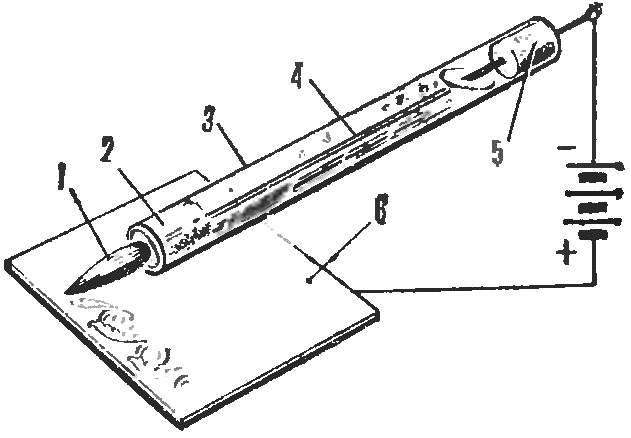
 Somehow I read in your magazine the article “rainbow on aluminum” and remembered a very convenient way of drawing figures and inscriptions on metal. Brush for these works is a glass tube filled with electrolyte which has the ability to seep through the hair insert one end of the tube. From the other end of the tube is inserted through the electrode connected with the battery of the flashlight and cover the metal surface. Touching the brush metal, we close the circuit, there occurs a galvanic covering.
Somehow I read in your magazine the article “rainbow on aluminum” and remembered a very convenient way of drawing figures and inscriptions on metal. Brush for these works is a glass tube filled with electrolyte which has the ability to seep through the hair insert one end of the tube. From the other end of the tube is inserted through the electrode connected with the battery of the flashlight and cover the metal surface. Touching the brush metal, we close the circuit, there occurs a galvanic covering.
It is better to procure a few of these brushes for each type of its coverage. For copper plating, the electrode must be copper, and the electrolyte composition is the following: sulfuric acid — 3.5 g, copper sulphate and 9.5 g distilled water — 100 g (pour acid into water!). Voltage — 1.5 V.
When Nickel plating the Nickel electrode, respectively, and the solution can be of two types: sulfate Nickel — 4.2 g, ammonium sulphate — 4.2 g, distilled water — 100 g, or instead of ammonium — sulfur double ammonium Nickel salt — 3G of sodium chloride is 0.8 g Good result is obtained when the current is 0.3 A and a voltage of 3.5 V.
Electrocity:
1 — hair insert, 2 — cotton filter, 3 — glass tube with electrolyte, 4 — electrode, 5 — tube, 6 — covered object.
The coated metal surface should be cleaned well with sandpaper, gasoline, washed with distilled water and wiped dry.
With the help of electricity you can quickly “paint” the small things, to execute decorative paintings and inscriptions.
A. IVANOV, Kursk