 For works in chemical laboratory hydrogen is produced usually by the reaction of zinc with hydrochloric acid (HCL). Sometimes you need a small amount of gas, and 1 g of zinc makes about 400 ml of hydrogen, so that, using part of it, the rest have to release into the atmosphere.
For works in chemical laboratory hydrogen is produced usually by the reaction of zinc with hydrochloric acid (HCL). Sometimes you need a small amount of gas, and 1 g of zinc makes about 400 ml of hydrogen, so that, using part of it, the rest have to release into the atmosphere.
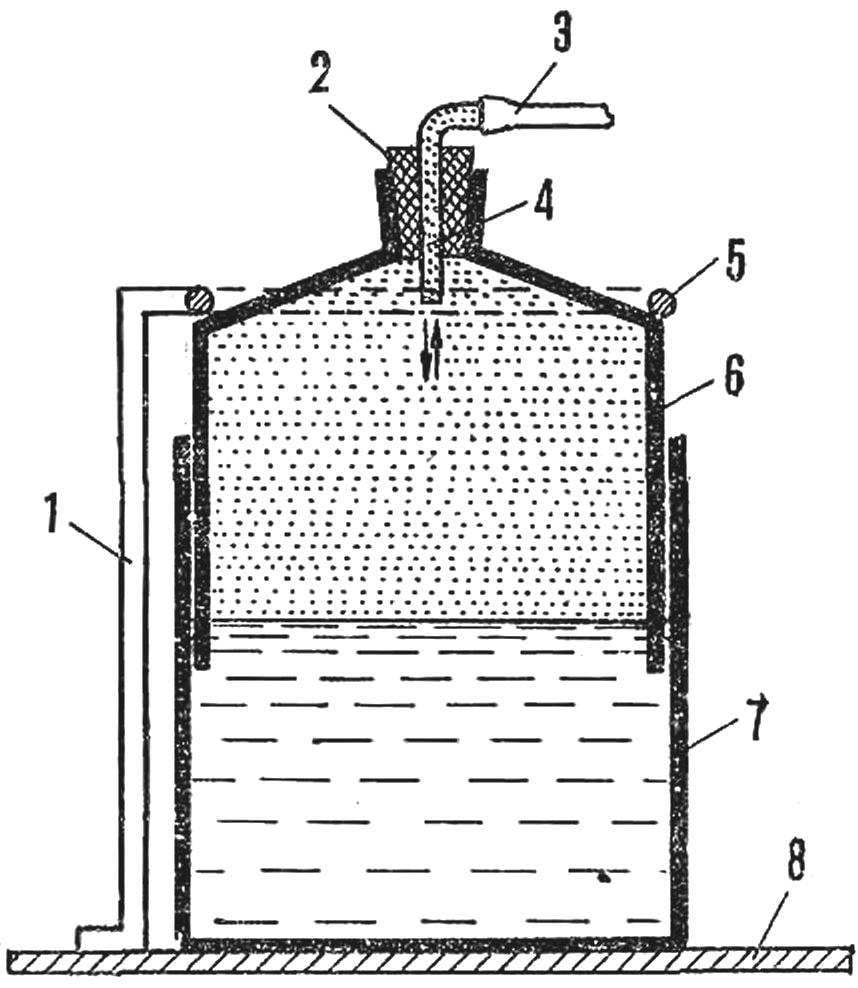
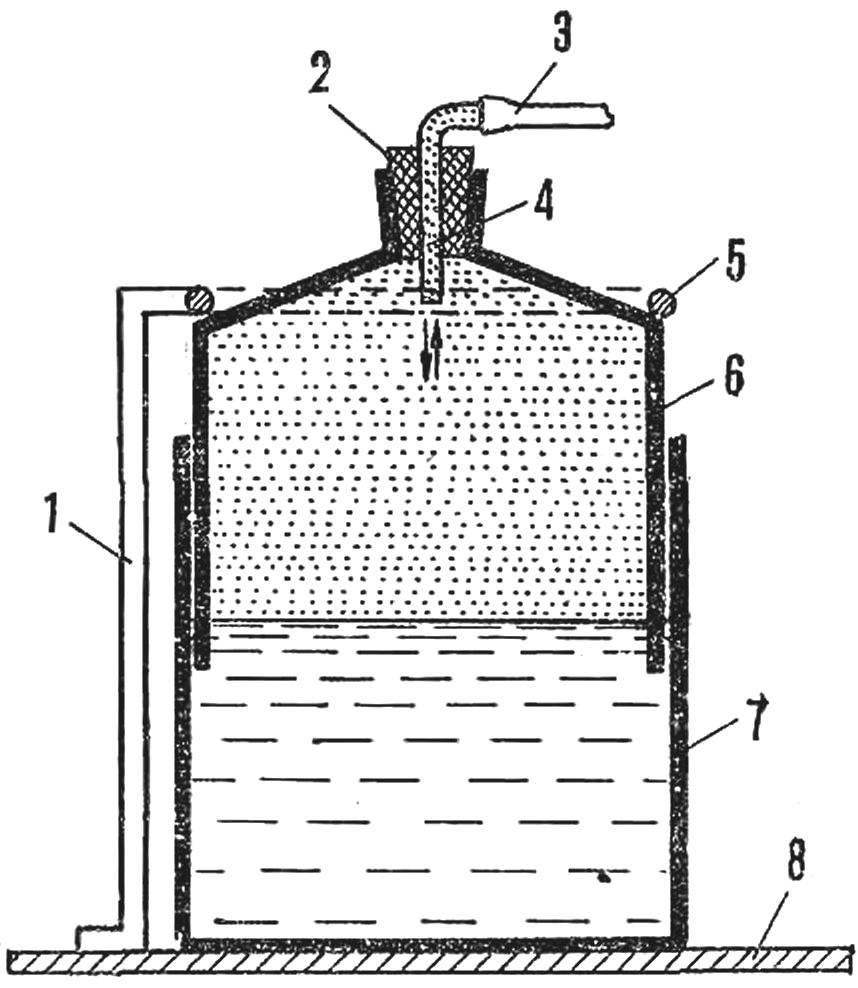
However, hydrogen can be stored in a simple tank. For this is the large plastic Bank 7 attached to the stand 8. Poured in a jar of water, the level of which is equal to the height of the bell, which will accumulate gas.
When the bell 6 no gas, it is submerged in water. The gas is introduced under a bell through the rubber tube 3 and another glass put through the tube 2. The bell is made of plastic cans of smaller diameter. Under the pressure of the incoming gas bell rises to a certain level, a limited focusing 5 on the rack 1. When the gasholder is filled with gas, the lower part of the bell should be immersed in water while the rubber tube is clamped with a clothespin.