Rust is enemy number one of almost any metal. The “red plague” that with enviable persistence and constancy turns hundreds of thousands of tons of sparkling high-grade, high-strength, alloyed steel into heaps of brown powder. A disease for which there are no barriers… But there are remedies for it: galvanic coatings, varnishes and paints, bitumens and mastics – all of them should in principle protect the metal. But in reality, everything is not so simple.
The problem of corrosion protection is very acute, for example, for motorists. It is well known that if certain measures are not taken, the car body can literally turn into a rusty sieve within four to five years. Often neither paint coatings nor mastics help, since the body has many closed cavities, recesses, pockets, boxes, in which road dirt and moisture, mixed with table salt, create excellent conditions for electrochemical corrosion. And with the modern thickness of automotive steel sheet, this leads to its very rapid failure.
But corrosion can not only be protected against with armor made of varnish or chrome, it can also be deceived by offering as bait such a tempting piece as a metal with a higher electrode potential.
Electrode potential? What does it actually have to do with metal corrosion? It turns out, the most direct.
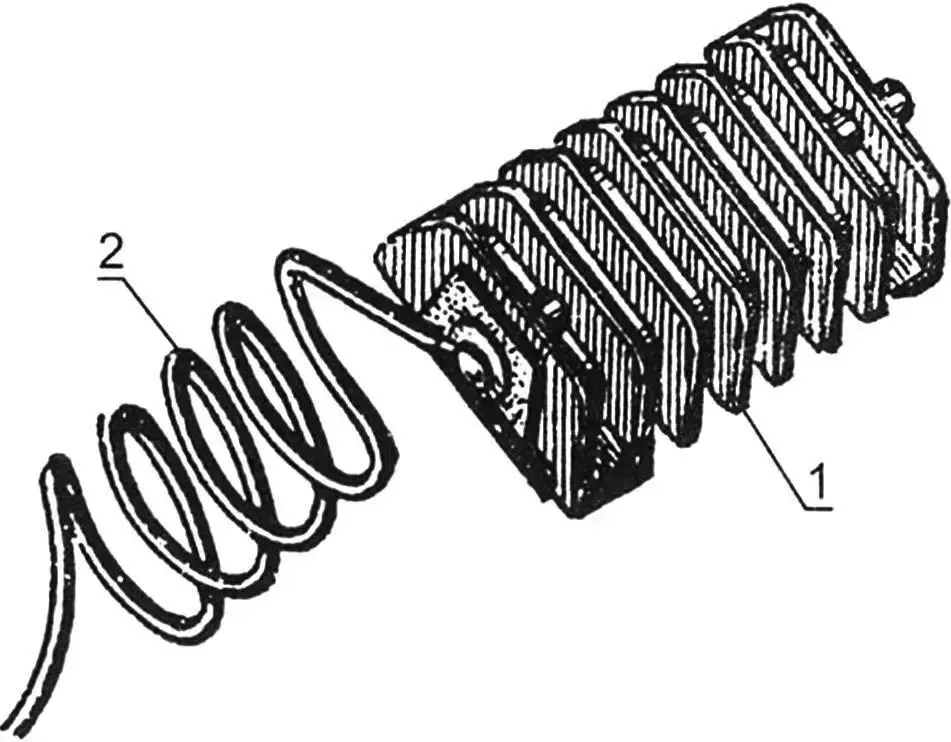
1 – finned zinc electrode; 2 – connecting wire
If two electrically connected metal electrodes are lowered into a vessel with an electrolyte, one of them will begin to dissolve, while the other will remain intact. So, it turns out that the metal with the higher electrode potential dissolves. This property of a galvanic pair made it possible to use the effect of cathode preservation to prevent electrochemical corrosion of the car body.
Shipbuilders have long used this principle to protect the inner part of the hold from corrosion – they place special metal anodes (made of metal with a higher electrode potential than the hull metal) inside the hull. Motorists have also adopted this method.
For anodic protection, finned (to increase surface area) pieces of zinc are used. With the help of permanent magnets embedded in them, they are attached to the most hard-to-reach and contaminated places on the body. Electrical connection is made with a multi-core wire: using screws, the zinc anode is connected to the body.
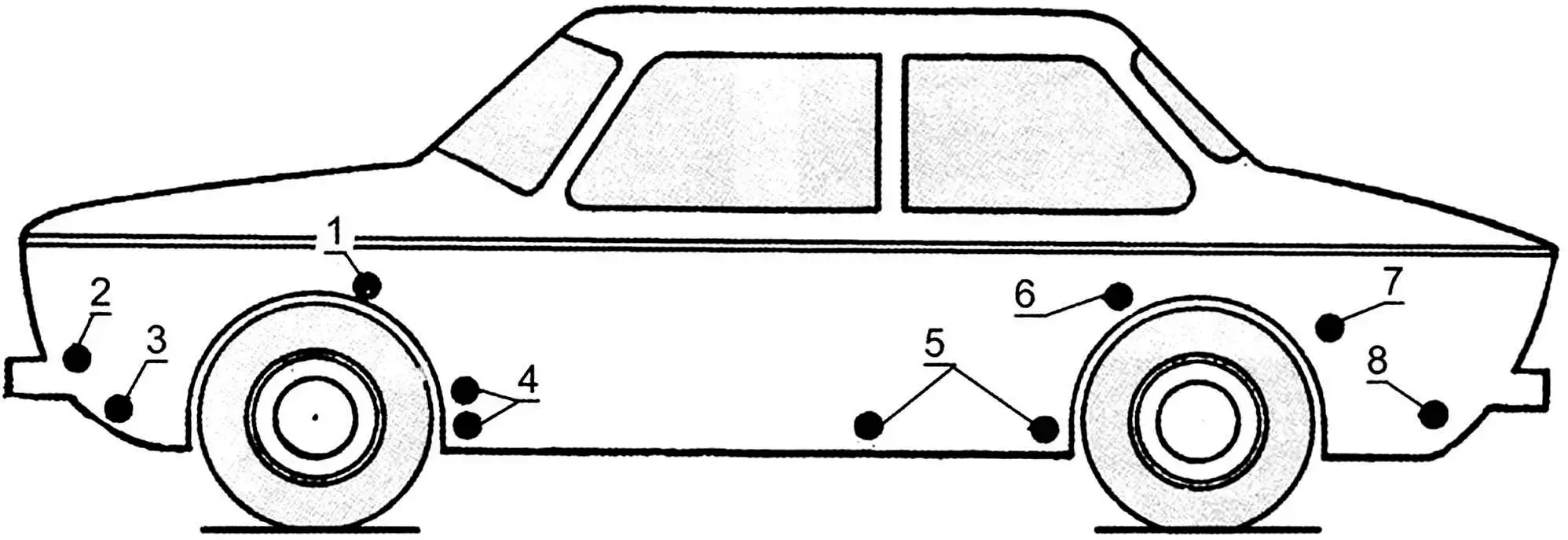
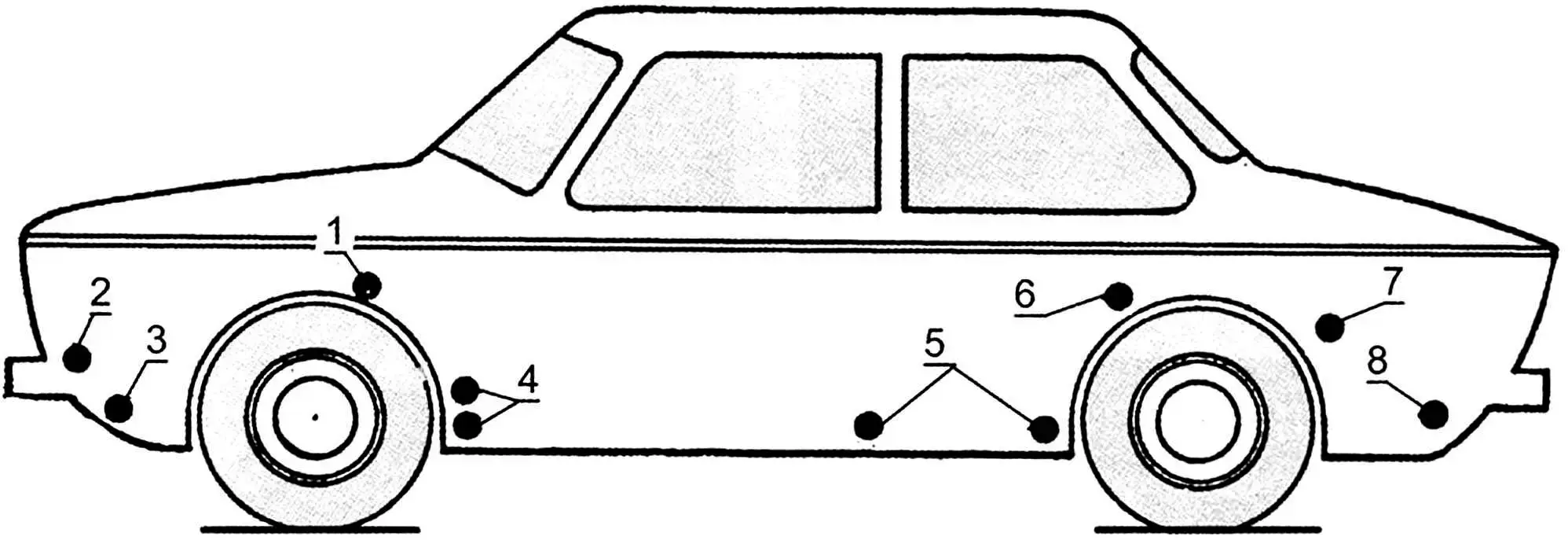
1 – box-shaped splash guard reinforcements; 2 – mounting points for headlight and fog light housings; 3 – lower part of front panel; 4 – cavities behind shield-reinforcements of front fenders; 5 – inner surfaces of doors; 6, 7 – front lower part of rear fender and wheel arch at the joint with fender; 8 – rear panel apron
Road dirt, moisture, and table salt collect on its fins, and the “zinc – steel” set begins to work like the well-known galvanic cell. When such a “battery” operates, the zinc anode dissolves, while the cathode in this case is not consumed.
The corrosion process resembles the operation of a galvanic cell, since steel is mainly an alloy of iron and carbon, that is, substances with different electrode potentials. When an electrolyte hits the surface of such an alloy, an electrochemical reaction begins between the grains of iron and carbon, accompanied by the dissolution of the anode (iron) and its transition into hydrates, and then into oxides.
However, the presence of a zinc electrode electrically connected to the base metal fundamentally changes the picture. In relation to both iron and carbon, zinc is a metal with a higher electrode potential, that is, it acts as an anode. Therefore, in the presence of an electrically conductive medium, which is practically always present on automotive body surfaces, the electrochemical reaction proceeds with the dissolution of the anode (zinc), while preserving the cathode, that is, the body metal.
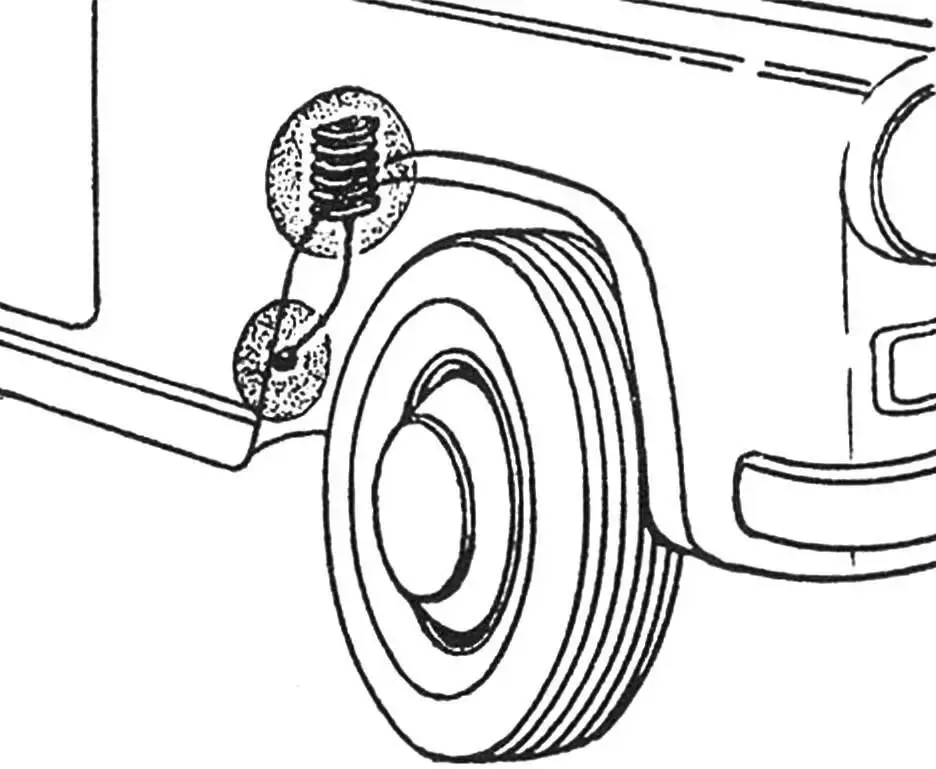
As experiments have shown, a zinc electrode the size of a matchbox lasts for 3 – 5 years.
Deceive the “red plague”. Offer it bait – a piece of metal with an electrode potential higher than that of steel. Corrosion will eagerly grab onto it, forgetting about your car body for at least three years.
(Based on materials from the magazine «Teknik for alla», Sweden)