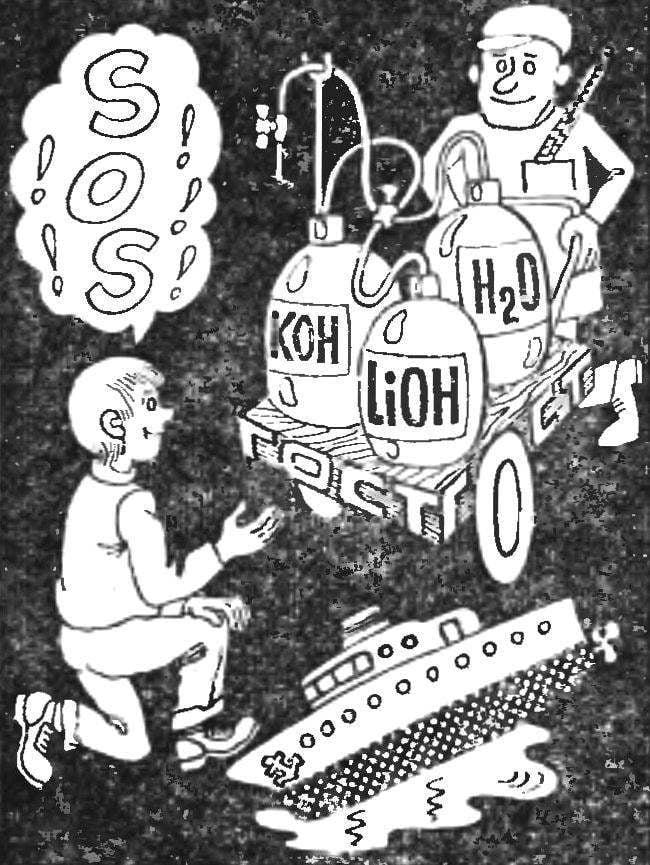
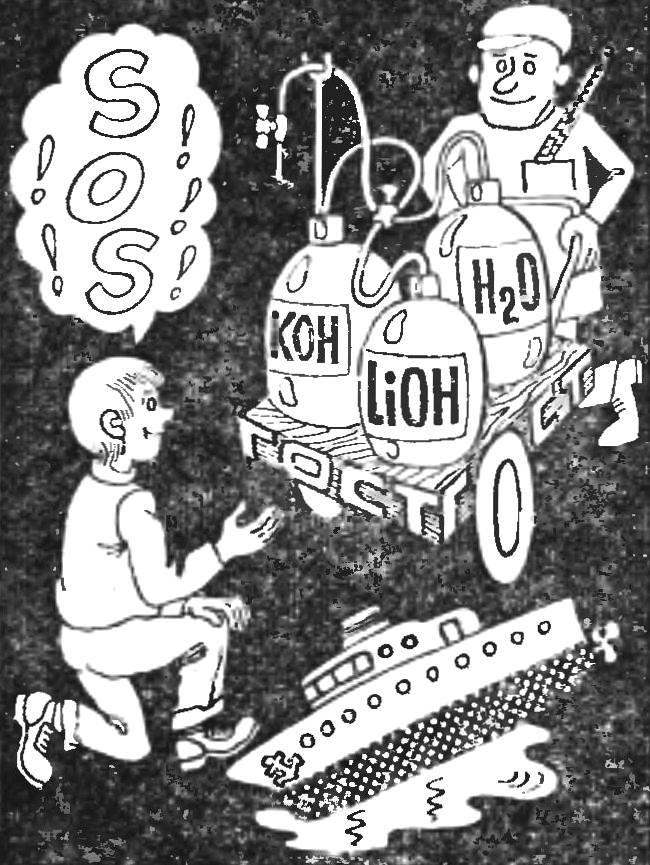
 How to make the electrolyte alkaline batteries? What are components, what should be the charging current, how long to charge? Electrolyte — aqueous solution of potassium hydroxide with a density of 1,19 — 1,21 g/cm3 with the addition of 20±1 g/l of lithium hydroxide monohydrate (Li0Н.H20). (This corresponds to GOST 9285-59. At -20±1°C or -40±1°C batteries should work for an aqueous solution of potassium hydroxide with a density of 1.26 — 1.28 g/cm3 without addition of lithium hydroxide.)
How to make the electrolyte alkaline batteries? What are components, what should be the charging current, how long to charge? Electrolyte — aqueous solution of potassium hydroxide with a density of 1,19 — 1,21 g/cm3 with the addition of 20±1 g/l of lithium hydroxide monohydrate (Li0Н.H20). (This corresponds to GOST 9285-59. At -20±1°C or -40±1°C batteries should work for an aqueous solution of potassium hydroxide with a density of 1.26 — 1.28 g/cm3 without addition of lithium hydroxide.)
In accordance with GOST 8595-57 the electrolyte level above the plates should be within 5 — 12 mm, according to GOST 9240-69 charging current in amperes numerically equivalent to 25% of the battery capacity. For example, if the battery capacity of 8 Ah, the charge current is equal to 2A (the battery brands 2ФЖН-8, 2KN-8 or 2НКН-8).
The duration of charging alkaline batteries is not dependent on the brand and capacity is 6 hours.
The battery life under optimal conditions: kidmannicole—not less than 1000 cycles, iron-Nickel — not less than 750 cycles.
V. MUKHIN, master of sports, Moscow